Lemon Battery
What to Do With a Lemon Besides Making Lemonade?
What You'll Need
- 18-gauge copper wire (smaller gauge will work too, but 18-gauge is stiffer)
- Wire clippers
- Steel paper clip
- Sheet of coarse sandpaper
- Lemon
- Help from an older friend or an adult
What To Do
1. Have your older friend or an adult strip 2 inches of insulation off the copper wire. Clip the 2 inches of bare wire with the clippers.
2. Straighten out the paper clip and cut about 2 inches of the straightened steel wire.
3. Use sandpaper to smooth any rough spots on the ends of the wire and paper clip.
4. Squeeze the lemon gently with your hands. But don't rupture the lemon's skin. Rolling it on a table with a little pressure works great.
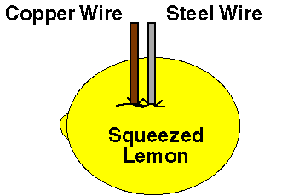
5. Push the pieces of the paper clip and the wire into the lemon so they are as close together as you can get them without touching.
6. Moisten your tongue with saliva. Touch the tip of your wet tongue to the free ends of the two wires.
Results
You should be able to feel a slight tingle on the tip of your tongue and taste something metallic.
What Happened
The lemon battery is called a voltaic battery, which changes chemical energy into electrical energy.
The battery is made up of two different metals (the steel paper clip and the copper wire). These are called electrodes, which are the parts of a battery where electric current enters or leaves the battery. The electrodes are placed in a liquid containing an electrolyte, which is a solution that can conduct electricity.
In a solution of water and an electrolyte, like the acid in the lemon, an excess of electrons collects on one end of the electrodes. At the same time, electrons are lost from the other electrode.
Touching the electrodes to your tongue closes the circuit and allows an small electric current to flow. A single lemon produces about 7/10 of a volt of electricity. If you connected two lemons together, you can power an inexpensive digital watch (uses about 1.5 volts). (Use a length of thin, flexible wire to connect the silver wire of one lemon to the copper wire of the other lemon. Then attach thin wires from the other two wires in the lemons to where a battery's positive and negative poles connect to power the watch.)
The tingle felt in your tongue and the metallic taste is due to the movement of electrons through the saliva on your tongue.
A Note About Lemon Energy
We've had some students do this project and then try to use the lemon "battery" to light a small flashlight's light bulb. The lemons did not work. Why? The reason is that the lemons produce only a very small current (about one milliamp). This is not enough electric current to light the bulb. Even with multiple lemons, the amount of current flowing through the wire is not enough. Though the voltage is high enough (1.5 volts with two lemons), the current is too weak. But it was a great experiment! Even if an experiment doesn't work, it helps us to understand why. Good work!!!
Article produced by The California Energy Commission. Online Information http://www.energy.ca.gov. All rights reserved. Used with permission

